[Originally published as Accelerated Radioactive Decay]
When I first heard about the idea that radioactive decay might vary from the smooth, constant-half-life behavior that is typically observed, I was more than a little skeptical. As a nuclear chemist, I am well aware of how much energy it takes to affect nuclear processes. Since those energies are not generally attainable except by using a particle accelerator, a magnetic containment system, or some other high-powered device, it seemed absurd to think that variable radioactive decay was anything other than the mad wish of those who didn’t like the conclusions of radiometric dating.
However, over the years, the data have convinced me otherwise. I have written a couple of posts about variable radioactive decay (see here and here), and it seems clear to me that it does happen, at least under some circumstances.
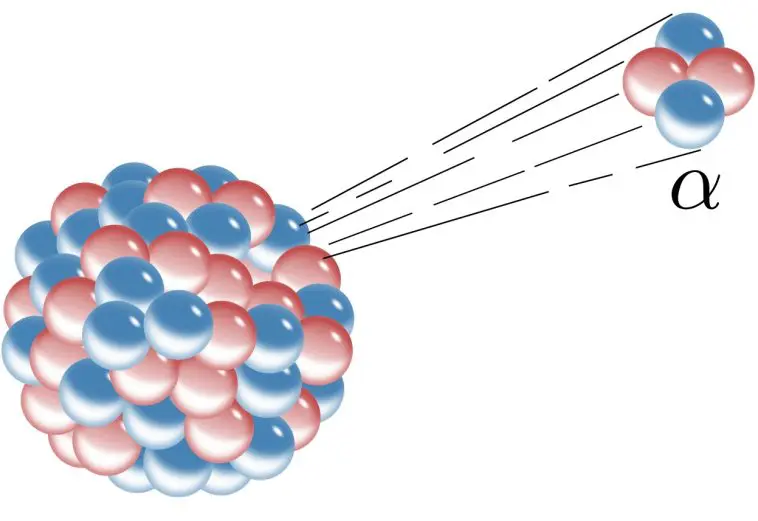
Recently, I came across another study on variable radioactive decay. It is a follow-up to a previous study, and it explores the alpha decay of uranium-232. As shown in the drawing above, alpha decay is one type of radioactive decay in which an unstable nucleus attempts to reach stability by spitting out two protons and two neutrons. Those four particles are bound together to form the nucleus of a helium atom, which for historical reasons is also called an alpha particle. It turns out that when uranium-232 does this, the resulting nucleus still isn’t stable, so a long series of further alpha decays occur, eventually producing lead-208, which is stable.
The authors of the study I am writing about weren’t interested in the subsequent decays. They looked specifically at the alpha decay of uranium-232. Under normal circumstances, this decay has a half-life of 69 years. This means if I start with 200 uranium-232 atoms, after 69 years, only half of them (100) will remain. The other half will have decayed away. If I wait another 69 years, only half of those (50) will remain. In another 69 years, half of those (25) will remain. In the end, this is typically how radioactive decay works: the number of radioactive atoms ends up decreasing by half over every half-life.
The results of the study seem to indicate that a tabletop device involving a laser and gold can end up decreasing the half-life of uranium-232 by as much as a factor of 435,494,880,000,000!2
What did the authors do to obtain such an incredible result?
They took a solution that contained dissolved uranium-232 ions and measured the number of alpha particles coming specifically from the decay of uranium-232. This is called the activity of the solution. They separated the solution into several samples and placed a gold target into each sample. They then fired one of four different lasers at the gold in each sample. After exposing the gold to the light from the laser for a while, they measured the activity of the sample. In each case, the activity was lower than it was prior to the laser burst, and the decrease was more than expected based on just the half-life of uranium-232.
Interestingly, the decrease in activity was different for each type of laser used. A copper vapor laser with a pulse that lasts for 20 billionths of a second produced the smallest decrease in activity, while a Nd:YAG laser with a pulse that lasted only 150 trillionths of a second produced the largest decrease in activity.
Over the course of an hour, the most effective laser decreased the activity of the solution by a factor of two. Normally, it would take 69 years for the activity to decrease by a factor of two. With the right laser, however, it happened in just an hour.
What do the authors think happened to produce this dramatic decrease in activity?
They think that when the laser hit the gold, it produced tiny particles (nanoparticles) of gold that dispersed in the solution. As the laser light continued to pulse, it interacted with those nanoparticles, producing a plasma effect that concentrated a large amount of energy into a tiny volume. This concentrated energy was enough to affect the process by which alpha decay occurs, speeding it up dramatically.
How dramatically did the decay speed up?
According to the authors, during the hour over which the solution was exposed to the laser, the light was only hitting the gold for a very short amount of time. This is because the laser pulses, and each pulse is only 150 trillionths of a second long. In between each pulse, there is “dead time,” where no laser light is being generated.
Based on the way this particular laser worked, the “dead time” lasted much, much longer than the laser pulses. As a result, the light was only hitting the gold for a grand total of 5 millionths of a second during the hour over which the sample was exposed. If the laser light really is what caused the decrease in activity, then those 5 millionths of a second of light resulted in the equivalent of a full half-life of radioactive decay. Thus, the authors contend that the laser reduced the half-life of uranium-232 from 69 years to 5 millionths of a second!
Do I believe the author’s results?
I am not sure. I think the most convincing part of the analysis is that each laser produced a different decrease in activity. That seems to indicate there is something about the laser light that is causing the uranium-232 to decay faster than it should. Based on this and the earlier study, however, it seems that the laser light is not enough. There has to be a metal, like gold, involved as well. Once again, that seems to point to the fact that there is a real effect going on here.
At the same time, however, I have my doubts about the methodology. It seems to me they didn’t test this as much as they could have.
According to the paper, the samples were exposed to the laser for only an hour. Why? It seems to me that they had the whole thing set up already. Why didn’t they expose the samples to the laser for an hour, measure the activity, and then do it for another hour? If the effect is real, they should see a further reduction in activity. Indeed, if each hour under the laser truly did produce a half-life worth of decay, they should have been able to reduce the sample to no detectable activity after about 10 such exposures. I contacted the lead author to ask why this wasn’t done, and I got no response.
Now, don’t take this criticism the wrong way. Overall, the data really do seem to indicate that this is a real effect. I hope someone else with the equipment can try to replicate this experiment and perhaps do several cycles of exposure to see how reproducible the effect is. Until that happens, I think the best I can say is that this is an interesting result that is worth investigating further.
References
- A. V. Simakin and G. A. Shafeev, “Accelerated alpha decay under laser exposure of metallic nanoparticles in aqueous solutions of uranium salt,” Physics of Wave Phenomena 19(1):30-38, 2011
- A. V. Simakin and G. A. Shafeev, “Accelerated alpha-decay of uranium isotopes induced by exposure of aqueous solution of uranium salt with gold nanoparticles to laser radiation,” Physics of Wave Phenomena 21(1):31-37, 2013