[Originally published as the first part of Radiometric Dating and the Age of the Earth]
Secular scientists date the Earth to about 4.5 billion years old by using selected radiometric dating results. Ultimately, what they call “deep time” serves as the very foundation of evolution theory. High school biology books openly acknowledge this necessary connection:
Evolution takes a long time. If life has evolved, then Earth must be very old. Geologists now use radioactivity to establish the age of certain rocks and fossils. This kind of data could have shown that the Earth is young. If that had happened, Darwin’s ideas would have been refuted and abandoned. Instead, radioactive dating indicates that Earth is about 4.5 billion years old—plenty of time for evolution and natural selection to take place.[i]
But as we show here, geologists do not use radioactivity to establish the age of certain rocks. They instead use selected radioactivity results to confirm what they need to see. As discussed in previous chapters, this viewpoint, being secular, contradicts God’s stated word in Genesis and even the Ten Commandments. There He wrote with His own hand that He created the heavens, Earth, sea, and all that is in them in six days (Exodus 20:11).
Belief in deep time rests upon evolution’s required time. That’s putting a lot of faith in something that can’t be tested through direct observation. After all, plenty of assumptions go into the calculations, as we’ll discuss later.
Keep in mind that while this chapter reviews the technical details behind radiometric dating, everything breaks down to two very basic but catastrophic “fatal flaws” which undermine the whole process.
The first fatal flaw is that it relies upon untestable assumptions.
The entire practice of radiometric dating stands or falls on the veracity of four untestable assumptions. The assumptions are untestable because
- We cannot go back millions of years to verify the findings done today in a laboratory, and
- We cannot go back in time to test the original conditions in which the rocks were formed.
If these assumptions that underlie radiometric dating are not true, then the entire theory falls flat, like a chair without legs.
 The second fatal flaw clearly reveals that at least one of those assumptions must actually be wrong because radiometric dating fails to correctly date rocks of known ages.
The second fatal flaw clearly reveals that at least one of those assumptions must actually be wrong because radiometric dating fails to correctly date rocks of known ages.
For example, in the case of Mount St. Helens, we watched rocks being formed in the 1980s, but when sent to a laboratory 10 years later for dating, these new rocks returned ages of hundreds of thousands to millions of years.
Similarly, some rocks return radiometric “ages” twice as old as the accepted age for earth. Most rocks return conflicting radiometric “ages.” In these cases, researchers select results that match what they already believe about earth’s age.
Overview of Radiometric Dating
Fossil remains are found in sedimentary rock layers. Layers of sediment form when various size particles (e.g., dirt, rocks, and vegetation) accumulate in places such as deserts, rivers, lakes, and the ocean. Most texts teach that it takes a long time for these sediments to build up, with older layers buried beneath younger layers. Fossils found in lower layers are deemed to be older than those in the upper layers. This is called relative age dating, which is the first step.
Next, evolutionary scientists use index fossils to help establish the relative ages of rock layers that are not directly related to one another and their fossils.
Index fossils are distinct fossils, usually of an extinct organism found in only one or a few layers, though that layer outcrops in many places—at least that’s the theory. These fossils help establish and correlate the relative ages of rock layers.
Index fossils typically have a short stratigraphic, or vertical, range. In reality, many index fossils occur above or below their expected ranges. In some cases, they turn up still alive today, but these mostly go unreported.
Evolutionists assume that the creature evolved somehow, lived for a certain time period, and then died out. Textbooks are correct when they state that relative dating provides no information whatsoever about a fossil’s absolute age. Nevertheless, most textbook writers and the scientists they rely on grew up with a belief in uniformitarian geologic processes.
The principle of uniformity is a philosophy and an assumption that the slow geologic processes going on today must explain the deposits of the past. They teach the motto, “the present is the key to the past.” It’s not. As any judge in court will attest, eyewitness reports record the past more accurately. Also, keen observations in the field testify that the sediments comprising the ancient rock layers were laid down catastrophically, not slowly over millions of years.
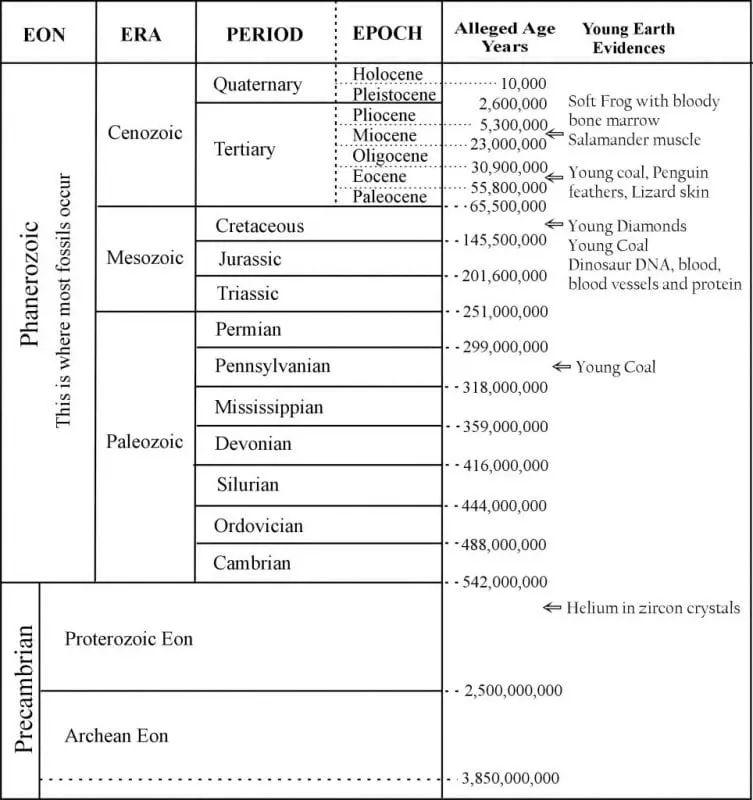
Today, the geologic time scale shows ages based on radiometric age dating. Many textbook authors consider radiometric ages as absolute ages. However, as you will soon learn, these techniques stray far from absolute dates, though they may reveal relative ages of some rocks.
The Age of the Earth
Today’s evolutionists base their age of the Earth on their interpretation of radioactive elements. They assign 4.5 billion years to earth based on the belief that earth itself evolved, so to speak, from a molten mass. But they cannot directly date the earth using selected isotopes because they believe all rocks have cycled over imagined eons, leaving no original rocks to test. They assume meteorites formed when earth did. Researchers age-dated a meteorite to sometime around the age they would accept. Thus, the earth itself has no direct evidence for its vast evolutionary age assignment.
The various rock layers are given names with assigned ages (Figure 1). Those who believe these ever-changing but always unimaginably old age assignments call each rock System a “Period.” The names help, but their age assignments derive from results chosen to agree with evolutionary time. To understand exactly why, we must first learn the basics of radioactive elements and of the techniques used when treating these systems of elements as clocks.
Many elements on the periodic table have radioactive forms. Stable atoms have a set number of protons, neutrons, and orbital electrons. Isotopes are atoms of the same elements with the same number of protons but different numbers of neutrons. Some isotopes are radioactive and others are stable. A radioactive nucleus is not stable. It changes into another element by emitting particles and/or radiation.
Footnote
[i] Kenneth R. Miller and Joseph S. Levine, Biology. (Boston, MA.: Pearson, 2006), p. 466.







