By Rachel Hamburg
Everything dies, and in fact, everything is dying this very second. Our bodies, our houses, our sun, our galaxy— the entire universe is slowly decaying away. The rate is exceptionally small, but ever present nonetheless. It’s a strange and dismal thought, especially as little lambs are born and Spring comes every year. Even so, this phenomenon should provoke us good scientists to ask at least two questions: how do we know the Universe is decaying away and why is it decaying? Fortunately, the second law of thermodynamics can answer half our questions, and if we know our Scripture, then the other half falls right into place.
Stating the Impossible
All in all, the second law of thermodynamics excites me even more than the first. It’s just as monumental, but more intuitive and relevant to our everyday world. It explains a lot about the relationship between heat and work, and in my opinion, is one of the strongest evidences against the Big Bang. It also leads to powerful conclusions about Nature, information, psychology, and beyond. Honestly, the second law goes far beyond what I could write here; but, as with all science, its fundamental concepts can be understood by anyone.
Interestingly, and unlike the first law, the second law of thermodynamics isn’t an equation. It’s a statement of impossibility. It defines how energy cannot be used and converted, and since it isn’t an equation, scientists came up with a variety of ways to word it. The two most popular are the Kelvin-Planck statement and the Clausius statement, named for the scientists who developed them. Together, they give a complete picture of what the second law means.
Let’s start with the least intuitive of the two: the Kelvin-Planck statement, which says,
“It is impossible for any system to undergo a process in which it absorbs heat from a reservoir at a single temperature and converts the heat completely into mechanical work, with the system ending in the same state in which it began.”
Now there’s a little “science-ese” in that, but it’s meaning is pretty simple. The key phrase here is: “It is impossible for any system to…[convert] the heat completely into mechanical work.” In other words, we cannot change a certain amount of heat into an equal amount of work, or more specifically, motion. In a steam engine, for example, heat is applied to water, which then boils and turns into steam. If put under pressure, the steam will push the blades of the turbine—thus, converting heat energy into motion. However, not all of the heat is converted into motion. Some cools during the process and does not lend its energy towards pushing the blades. Because of this, steam engines are not 100% efficient. Engineers have never been able to invent an engine that converts every single bit of its energy into motion—and according to the second law of thermodynamics, they never will. It is absolutely impossible for any thermodynamic system, such as a steam engine, to be 100% efficient because energy is always lost during the process.
The Clausius statement is much simpler, saying, “It is impossible for any process to have as its sole result the transfer of heat from a cooler to a hotter body.”
Meaning, heat will not spontaneously flow from something cold to something hot. Hot food, for example, cools down if left to itself. The heat transfers from the food (the hot body) to the air (the cool body). However, cold food will never heat up all by itself. Heat in the air may transfer to the cold food, warming it a little bit; but in a vacuum (an empty space without air), there is no warm air to heat the food up. If we wanted heat to flow the opposite way, then we’d have to force it by using a heat pump, compressor, or similar type of machine. That wouldn’t be a natural, spontaneous process. Man had to “manipulate nature,” if you will.
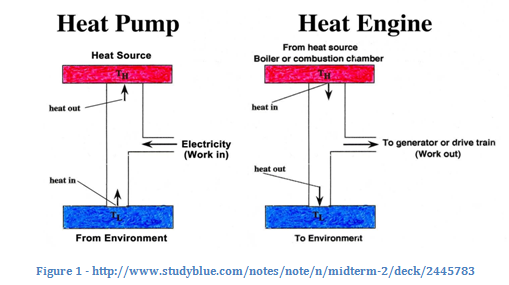
Regardless, these two statements, though appearing different, illustrate the same law. Take a look at the diagram. The heat pump corresponds to the Clausius statement, and the heat engine to the Kelvin-Planck statement. In the heat pump, heat is flowing from cold to hot only because work is being added. In the heat engine, not all the heat is converted to work; some cools down and goes out to “the environment.” These two statements both explain the relationship between heat and work–just from different perspectives.
The second law probably doesn’t shock you . . . because we experience it everyday. Heat dissipates, gases flow from high pressure to low pressure, gases tend to mix with liquids. Basically, the second law says that nature proceeds from order to disorder, neat to messy, high energy to low energy. As I mentioned, there are a variety of ways and examples we can use to explain the second law, but the law itself is simply the principle of continual decay over time.
The Arrow of Time
In the 1850s, while studying the second law of thermodynamics, the German scientist Rudolf Clausius (of the Clausius statement) coined the word entropy, meaning “the measurement of disorder.” Essentially, every time you see the word entropy, you can replace it with the word disorder. Although the second law doesn’t have an equation itself, scientists can calculate its effects on thermodynamic bodies by measuring their entropy before and after the process. Through innumerable experiments, scientists have shown that entropy implies a few interesting things about our world:
Any process either increases the entropy of the universe or leaves it unchanged. All natural processes are irreversible and tend toward increasing disorder. The total entropy of the universe always increases. Nature proceeds from low entropy to high entropy.
So let’s get this straight. In nature, the past is more orderly and “perfect” than the present. As time goes on, nature continues to break down. Hmm, why does that sound so . . . Scriptural. If we adhere strictly to science, then starting with a created, “very good” world makes much more sense than starting with an explosion that just happened to form the laws of physics and everything we see!
Well, atheistic scientists are truly well aware of this problem. By observation and experimentation, they know that the early universe had very low entropy—but they just don’t know how to account for it. In fact, it’s one of the unsolved questions in physics! If you take a look on Caltech physicist Sean Carroll’s website, he has this posted:
“Does inflation [the rapid expansion of the universe caused by the Big Bang] explain the low entropy of the early universe? Not by itself, no. To get inflation to start requires even lower entropy initial conditions than those implied by the conventional Big Bang model. Inflation just makes the problem harder.”*
There it is, folks. Inflation according to the Big Bang only confuses things. But our God—who stretched out the heavens, laid the foundations of the earth, and formed man from the dust of the ground—did not lie to us. He told us all about Creation in the very first chapters of Genesis, and nothing He said contradicts anything in the natural world.
So I hope now you can see why I find the second law of thermodynamics so amazing. Still, there are so many more applications and seeming contradictions about the second law that I’m hoping to write about next time. But until then, I’ll leave you with the words of the great scientist Sir Arthur Eddington—
“If someone points out to you that your pet theory of the universe is in disagreement with Maxwell’s equations—then so much the worse for Maxwell’s equations. If it is found to be contradicted by observation–well these experimentalists do bungle things sometimes. But if your theory if found to be against the second law of thermodynamics I can give you no hope; there is nothing for it but to collapse in deepest humiliation.”**
* http://preposterousuniverse.com/eternitytohere/faq.html
** http://web.mit.edu/keenansymposium/overview/background/