Overview
While everything around us gives the appearance of being solid and compact matter; the truth is, more than 99.9999% of every material that exists in our universe is made up of absolutely NOTHING!!!
Furthermore, what we perceive as the surface of a solid rock, the cool flow of a stream of water, or gentle caress of a summer breeze actually involves no physical contact whatsoever.
Over 99.9999% of every material in our universe is NOTHING; and, we can never actually ‘touch’ any other object in existence.
The way in which our physical universe is ‘put together’ on the very small or subatomic level, provides some fascinating insight into the material design of our very existence. We also find that, as we examine the nature and behavior of smaller and smaller particles, many questions arise which cannot be easily answered by conventional scientific means.
Likewise, there are four forces at play in our universe that obey very specific mathematical laws. The forces are measurable and predictable and are at work all around us constantly. They are known as:
Gravity
Magnetism
Strong Nuclear Force
Weak Nuclear Force
We can calculate and predict many physical properties & behaviors associated with each of these forces; however, we do not understand HOW any of these forces actually works!!!
This article will focus primarily on magnetism – specifically electromagnetism at the atomic level and the strong nuclear force.
Helium (He) – A View at the Atomic Level
In order to look at some of the most basic common components that make up all of the matter around us, we need to enter a ‘world’ that is incredibly tiny. The size of this miniscule ‘world’ is many orders of magnitude smaller than even our most powerful visual (light-based) microscopes can peer into – and far, far tinier than we can easily conceive of logically.
Because of the vast difference in size between the ‘atomic’ world and the ‘macroscopic’ world (i.e. the ‘world’ in a size to which we are accustomed to living) we will use some comparisons and analogies to try to make sense of the sizes and relationships we will be examining in this article.
Let’s begin by getting a feel for the size of a typical atom. We could simply say that one He (helium) atom is approximately one ten-billionth of a meter in diameter (a meter is just under 40 inches in length)1. Since most of us can’t really comprehend the value of a number like ten-billion, we need to provide some kind of point of reference. One way to do this is to compare the size of the He (helium) atom to something we CAN see…
The smallest single object that can be seen clearly with the human eye is about 0.1mm (0.1 millimeters, or 1 ten-thousandth of a meter)2. This is about the diameter (or thickness) of a coarse human hair3. If you drew a straight line across the diameter of one human hair you could fit about 100,000 He (helium) atoms from end-to-end!!!
That size comparison, while astounding in that it shows just how many atoms there are in such a small space, may still be difficult to fathom because it is so difficult for us to find a common point of reference for something that is one-hundred-thousand times smaller than the width of a human hair. So, let’s blow the same comparison up to our ‘macroscopic’ world to see if that helps…
If we were to increase the size of the He (helium) atom to the size of a BB, which is something we can see with our unaided eye, then we can compare that to the diameter of a human hair at the same scale – that is 100,000 BB’s placed end-to-end. The diameter of a modern steel (plated or unplated) BB is about 4.45mm (0.175 in)4. If we placed one-hundred-thousand BB’s end-to-end they would extend for 0.45km (a little over 1/4 mi).
The comparison above makes a lot more sense in our ‘macroscopic’ world. If we go to a map and find any two locations that are 0.45 km (or just over 1/4 mi) apart and draw a circle with those two locations as endpoints of the diameter, that would represent the width of one human hair, if a He (helium) atom was the size of a BB!!!
Now we are beginning to get a real sense of just how small the ‘world’ of atoms really is!!!
Now, as fascinating as everything we’ve looked at so far may seem, this is going to seem almost trivial compared to what we are about to explore…
Remember at the beginning of this article we made a very bold statement that ‘more than 99.9999% of every material that exists in our universe is made up of absolutely NOTHING’? Well, most of that ‘nothing’ is inside of every atom!
Let’s begin by examining the basic structure of the atom (for the purpose of this article we’ll keep things really simple).
Everything in the universe is made up of elements, and every element is made up of atoms. Every atom has a nucleus and one or more electron shells. With the exception of the lightest element, H (hydrogen), every atomic nucleus is comprised of protons and neutrons (the hydrogen atom has only one proton in its nucleus). Electrons orbit each atomic nucleus within the electron shells. Each of these components of the atom is defined below:
Proton: Positively charged particle in the nucleus of an atom
Neutron: Neutral particle in the nucleus of an atom
Nucleus: The center of an atom
Electron: Negatively charged particle orbiting an atomic nucleus
Electron Shell: One or more regions in which electrons reside while in orbit about a nucleus
For the purpose of this article we will not be examining electron shells any further other than to note that the larger an atom is (i.e. the more electrons it has) the more electron shells it will have. Electrons organize themselves in a very orderly and predictable manner within these shells based on a standard orbital shell model5. When we examine the diameter of a particular atom in this article, we will only be concerned with the outermost electron shell.
A further note to clarify electron shells: An electron shell is NOT a physical object as we might think of, such as an ‘egg shell’. Instead, it is simply a region of subatomic space about the nucleus of an atom where an electron orbits – much like the orbit of a planet about the Sun except that an electron orbit takes place in 3 dimensions (this is why it is called an orbital ‘shell’ and not an orbital ‘ring’).
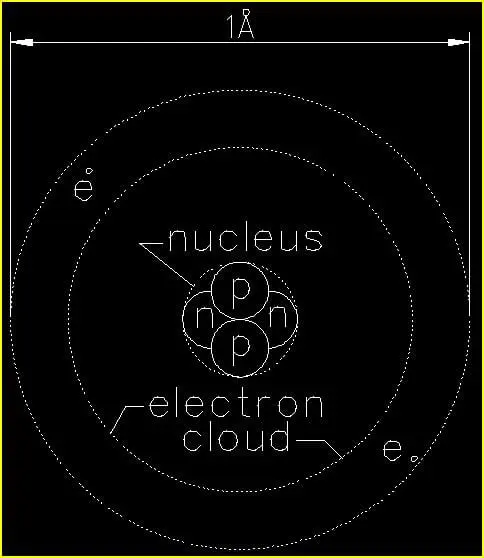
Figure 1 is a depiction of a single He (helium) atom. Since helium is a very stable element in its natural state and does not easily react with other chemicals, it commonly used as the foundational atom for determining the sizes of all other atoms and atomic structures in their many forms.
The diameter of a single He (helium) atom is approximately one-ten-billionth of a meter (1/10,000,000,000 m). As we just saw in our example comparing a He (helium) atom to a human hair, the numbers get extremely small on the atomic level. So, we have another unit of measure that makes things a lot easier for us. This unit is the Ångstrom.
1Å (Ångstrom) is approximately the diameter of a single He (helium) atom.
Rather than make things very confusing by switching back and forth between Ångstroms and meters and feet…etc, we will simply refer to all sizes in Ångstroms from this point forward until we need to scale our example again to macroscopic size for clarity.
The first thing we want to note in figure 1 is that the atom is NOT drawn to scale. In reality the electrons orbit the nucleus at a far greater distance than shown. We will discuss this in a bit when we examine the Fe (iron) atom.
Now, let’s examine the He (helium) atom from the inside out…
In the nucleus we find two (2) protons (p) and two neutrons (n). These are four separate subatomic particles that perform two different functions in the nucleus of every atom. For the scope of this article we will focus only on the function of the protons which carry a positive (+) charge much like the anode of a battery. Neutrons carry no charge. For this reason we sometimes write the symbol for proton as (p+) and for neutron as (n°).
Now remember, these are the most basic building blocks of everything. These too are made up of even smaller particles which we will not discuss in this article. However, their mention here is important because later we will be addressing just how much of matter is actually ‘nothing’, and more particles means more ‘nothing’ between those particles.
Because there is no ‘glue’ or latching mechanism that holds the particles together in the nucleus, we have to consider another force at work here. This force is known as the strong nuclear force. What this ‘force’ is cannot be physically ‘seen’; but, its effects can definitely be observed. We also know that it takes a tremendous amount of energy to break even one particle out of the nucleus of an atom; so, the force is indeed quite strong. But where does it come from? How did it get there? How does it keep holding all of those atomic nuclei in such tight formation?
The answer is, from a purely secular scientific perspective – no one really knows!!!
This would be a good place to pause in the reading of this article and turn to
Colossians 1:16-17 in your Bible and read that passage
(the ‘Him’ refers to Christ).
Moving outward we find two electrons (e) orbiting the nucleus. While protons and neutrons are basically the same size, electrons are about 1/10 the diameter of a proton. Electrons move VERY quickly – about 1% of the speed of light6 in a ‘close’ orbit about the nucleus as in the case of a He (helium) atom. Moving this quickly about the nucleus the orbiting electron(s) give the appearance of a smooth surface when examined up close. In our macroscopic ‘world’ everything that ‘seems’ to be solid is actually the result of an untold number of incredibly fast moving electrons giving the mere ‘appearance’ of solid matter!!!
Another characteristic of the electron is that it is negatively charged. Sometimes we write the symbol for the electron (e) as e–. Just as electricity flows between the anode (+) and cathode (-) of a battery; or, just as opposite ends (North and South) of magnets attract one another, electrons – having a negative charge – are attracted to the positively charged protons in the nucleus of an atom. The force that causes attraction between all of the objects mentioned in this paragraph is electromagnetism.
Electromagnetism is an attractive (magnetic) force that is produced in the presence of an electrical field. All charged particles in motion generate an electrical field.
The electromagnetic force is about 1/100 as strong as the strong nuclear force we previously mentioned that binds the particles together in the nucleus of the atom. The weakness of this force coupled with the sheer velocity of the electron brings us to another incredible fact about the structure of the atom…
Even though an electron weighs almost 2000 times less than just one proton7, the combined mass of even the largest atomic nuclei cannot even pull this highly energetic particle very close. In fact, in figure 1 if the entire He (helium) atom nucleus was the size of pea then the two electrons would be too tiny to be seen with the naked eye, and would orbit the nucleus in a ‘cloud’ with an average diameter of a football field from end zone to end zone!!!
Now as with the strong nuclear force which binds the particles of the nucleus together, the electromagnetic force cannot be physically ‘seen’. There is no electromagnetic ‘string’ that ties the electron to the nucleus of the atom. However, just as magnets are attracted to one another or repel one another by an invisible force, so too do protons attract electrons. Likewise, protons repel protons and electrons repel electrons. So, the forces at work to hold all of these particles in place are very significant – even though no one can actually ‘see’ them.
By the way, it’s the repulsion of electrons against each other which gives the ‘appearance’ that objects in the ‘macroscopic’ world actually touch each other. In fact, at the atomic level particles of adjoining objects come infinitesimally close to one another; however, due to electromagnetic repulsion of electrons against electrons (both are negatively charged and ‘like’ charges repel) they do not make any ‘contact’ (the sheer velocities of the electrons would make this impossible anyhow). So what we are ‘feeling’ whenever we touch a surface or hold an object is actually the interaction between the electromagnetic fields of the atoms in our bodies and the atoms of the object.
It is physically impossible for any two objects in the universe to ever actually ‘touch’ each other.
Again, while we can calculate and predict the behavior of objects influenced by electromagnetic forces, in the most basic of terms secular science has no real explanation for what it actually IS.
(Refer again to Colossians 1:16-17. Remember: ‘Him’ refers to Christ.)
Iron (Fe) – A Macroscopic Model
While it’s nice to examine the He (helium) atom because it is the smallest atom containing all three (3) of the basic subatomic particles being discussed in this article, very few of us can really relate to helium as an everyday material. Of course most of us have carried around helium-filled balloons at carnivals or sent them to someone for a special occasion. A few of us may have even inhaled a little helium gas to make our voices sound like a cartoon character for a moment. Quite honestly, however, as a colorless, odorless, and tasteless gas, helium is not the ideal material to use when we really want to make an impression on someone who wants to know just how much of ‘nothing’ is in everything. After all, in some sense we kind of think of many gases that are in the air around us everyday as being almost ‘nothing’.
Now if we really want to use a material that we can get a ‘feel’ for, we can use Fe (iron)…
If someone were to hand you an iron bar and ask you to describe it you might use words like: hard, solid, heavy, dense, etc. One expression you would probably never think to use is ‘mostly nothing’ – and yet, that is EXACTLY what that iron bar is!!!
The Fe (iron) atom has 26 protons and 30 neutrons its nucleus, which is orbited by 26 electrons. Using our ‘macroscopic world’ analogy let’s make one proton (and therefore one neutron as well) the size of a small pea. This means the nucleus would be made up of 56 peas in our macroscopic model. This would be about enough to fill a tennis ball of space.
Using this same scale our ‘electrons’ would be about the size of a small poppy seed.
If we were to construct a still model using this scale to represent the Fe (iron) atom we would need about the amount of space found across the outside of the short diameter of the entire Indianapolis Speedway (see figure 2)!!!
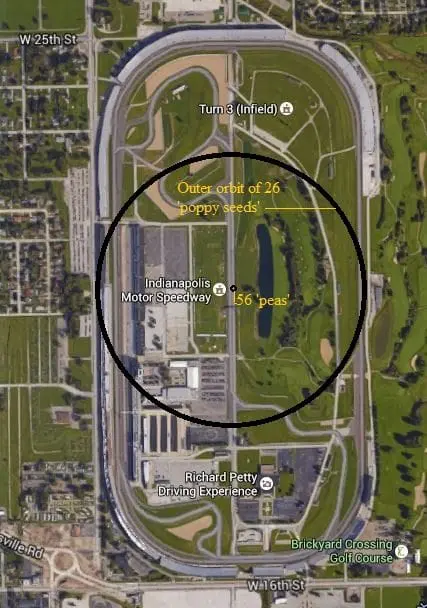
Just imagine…only 26 poppy seeds orbiting a nucleus about the size of a tennis ball at a distance of 5/16 mile (making a 5/8-mile diameter ‘atom’) while all the rest of that ‘space’ is just NOTHING!!!
That is EXACTLY what you are holding in your hand each time you pick up an iron bar, or an iron chair, or anything else made of iron.
The amount of ‘matter’ in a single iron (Fe) atom is equivalent to having 56 green peas and 26 poppy seeds in a space the width of the Indianapolis Raceway – everything else is NOTHING!!!
…and yet, this is what we call ‘solid matter’.
Just imagine what would happen to that ‘solid matter’ if the electromagnetic force that keeps the electrons in orbit about those nuclei were suddenly ‘switched off’!!!
Even more sobering…just imagine if all of the forces that hold the subatomic particles in place were suddenly switched off. Our entire universe would instantly come ‘unglued’ and appear to ‘vanish’ because it is, in fact, almost all NOTHING.
What makes the iron appear ‘solid’ to us is the poppy seeds (electrons) orbiting the peas (nucleus) so quickly that the outermost ones appear to form a ‘surface’.
By the way, you’ll notice that those ‘peas’ in the ‘nucleus’ of our Fe (iron) atom have spaces between them too. We also know (although not covered in this article) that each of the ‘peas’ (protons and neutrons) are made up of at least 3 separate smaller particles with at least some space between them and a ‘force’ that binds them together.
…and where did all of that energy come from anyway that gives protons and electrons their charges, makes electrons whiz around subatomic space endlessly orbiting, wiggling & spinning (we didn’t mention they ‘wiggle’ & ‘spin’ did we?), all the while holding all together in just the right way so that the same building blocks make all of the 94 naturally occurring elements8 that comprise all that we see, handle, use, and are?
(Once again…Refer again to Colossians 1:16-17. Remember: ‘Him’ refers to Christ.)
God’s Integral Hand
In John 1:1-3 we learn that Christ, ‘The Word’, made EVERYTHING. Nothing that exists came into being without Him. He is the same Creator we find in Genesis Chapter 1 who has created all things both in the heavens and in earth. Knowing this, it makes perfect sense that His Hand is upon everything that He has created, and the signature of His Handiwork is found in everything that exists.
The signature of God’s Handiwork is found in everything that exists.
Hebrews 11:3 tells us that even TIME ITSELF (the ‘worlds’ or ‘ages’) was created (‘framed’) by Christ, ‘The Word’, out of absolutely nothing – a concept which our mortal minds can scarcely fathom. Furthermore, in Paul’s letter to the Colossians 1:16-17 we read that everything was made BY Christ and FOR Christ. Not only did He Create all things; but, by Him ALONE all things are held together (consist).
In His perfect wisdom, God has allowed a vast ‘volume’ of ‘nothing’ to remain in everything to demonstrate His continual and active Hand in binding everything together.
Summary
As we seek to discover the secrets of the ‘inner’ universe we must build larger and more powerful devices to generate enough energy to literally ‘smash’ subatomic particles into one another9. It is indeed an amazing paradox that the tinier the particles become (and thus the close we get to the ‘force’ that binds them together) the more power we must generate to tear them apart!!!
This is just one more in the nearly infinite list of verifications that God is not only the Creator, but the Sustainer of all that exists – and the Bible makes it clear that the Person of God charged with Creating and Sustaining it all is Christ Jesus.
In His Love,
Dr. Jack L. Burton
-Hebrews 11:1-3
End Notes
(1) Metric Conversions. Meters to Inches Table.
http://www.metric-conversions.org/length/meters-to-inches-table.htm
(2) Wonderopolis. What is the Smallest Thing You Can See?
http://wonderopolis.org/wonder/what-is-the-smallest-thing-you-can-see/
(3) Schwartzkopf International Hair Dictionary. Hair Quality.
http://www.schwarzkopf.international/sk/en/home/hair_repair/tips_and_tricks/hair_science/hair_dictionary.html
(4) Airgun Depot. Pellets vs. BBs.
http://www.airgundepot.com/pelletsvsbbs.html
(5) Electron Shells and the Bohr Model.
https://www.boundless.com/biology/textbooks/boundless-biology-textbook/the-chemical-foundation-of-life-2/atoms-isotopes-ions-and-molecules-50/electron-shells-and-the-bohr-model-276-11409/
(6) TutorVista.com. Bohr’s Theory of Hydrogen Atom. Subtopic – Velocity of electron in a stationary orbit… http://www.tutorvista.com/content/physics/physics-iv/atoms-and-nuclei/bohrs-hydrogen-atom.php#velocity-of-electron-in-a-stationary-orbit-substituting-the-expression-for-r-in-the-equation
(7) Texas A&M University. Chemistry.
https://www.chem.tamu.edu/academics/fyp/educator_resources/isotopes_introduction.php
(8) Windows to the Universe. Earth. The Periodic Table of the Elements. http://www.windows2universe.org/earth/geology/periodic_table.html&edu=high
(9) How Atom Smashers Work.
http://science.howstuffworks.com/atom-smasher2.htm
References
(1) Merriam Webster Online Dictionary. Electromagnetism.
http://www.merriam-webster.com/dictionary/electromagnetism
(2) Iron: radii of atoms and ions.
https://www.webelements.com/iron/atom_sizes.html
(3) Encyclopaedia Britannica. Electron: Subatomic Particle.
http://www.britannica.com/science/electron
(4) Periodic Table by WebElements.
https://www.webelements.com/
Additional Reading
(1) Shell Model of Nucleus.
http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/shell.html
(2) https://thecreationclub.com/more-proof-youre-not-accidental-chemistry/
(3) https://thecreationclub.com/an-evolutionary-quandary/
Notes
1. All Bible quotes in this article are taken from the KJV.
2. Bible passages in double quotes (“) appear exactly as found in the KJV.
3. Bible passages in single quotes (‘) have been modified for emphasis or ease of reading only (such as capitalization of pronouns referring to God, bolded text, or modernized punctuation, etc.) without altering the actual wording of the text.
Disclaimer: While to the author’s knowledge all of the information cited in the referenced material related directly to the content of this article is correct and accurate, the author in no way endorses any of the cited references in their entirety – especially where the material contained therein is in direct contradiction with the Creation account given in the Word of God.








Hello:
Great article but I found myself wincing now and then when reading your description of electrons “orbiting” around the atomic nucleus. That analogy was abandoned many years ago. These days we have good evidence that electrons live in discrete energy levels but bound by specific volumes (shapes if you will) that look like a sphere (s orbitals), dumbbell (p orbitals), torus (d orbitals) and even more complex shapes. So the situation is even more complicated than just a simple orbit (we still call the shapes “orbitals” as a throwback to those simpler days of understanding electron movement as an orbit).
Thanks for the article though. I did enjoy it.
Dr. Ed Neeland
Chem Professor
Awesome! You made my day with this great article! I learned so much about our Creator! Stay blessed and keep up the good work.
Jennifer,
Thanks for your kind words and encouragement. It is always such a blessing to know that the Lord is using me to bless others with some of the vast amount of evidence He offers of His Handiwork in Creation.
In His Love,
Dr. Jack L. Burton
-Hebrews 11:1-3
Dr. Neeland,
Thanks for your comments and for your (complimentary) review 🙂 .
Please accept my apologies for making you ‘wince’ with my use of the ‘traditional’ orbital shell model in my analogy for this article. In truth, I had considered making reference to the probability clouds for the various ‘shells’ based on more recent evidence; however, the more I thought on how do address this ‘simply’ to such a wide audience the more I found it impractical to do so. If you have any suggestions on how I might do this in any future articles I write on chemistry and the atomic model, please feel free to share them with me.
Since the primary focuses of this article are on the vast amount of ‘nothingness’ found in all matter and the unknown nature of the attractive forces that bind all things together (if we exclude the Lord from the equation), the orbital shell model serves adequately for this purpose.
On the other hand, as we learn more about subatomic particle behavior and find that it is much more complex than we had ever imagined before it only lends further credence to the irreducible complexity which marks the handiwork of the Creator’s magnificent design.
In His Love,
Dr. Jack L. Burton
-Hebrews 11:1-3