[Originally posted as Carbon-14 Dating and Biblical History]
Carbon dating assigns ages to once-living materials such as wood, bone, teeth, and shells. Evolutionary researchers do not use it to age-date rocks. It begins by measuring the ratio of radioactive versus stable versions of an element. Carbon dating works by basing an age calculation on the ratio of radioactive carbon (14C) to normal carbon (12C) in the atmosphere before nuclear bomb testing to the same ratio in the sample. Carbon-14 decays to nitrogen. Using a formula that compares that ratio to a standard modern ratio produces a “percent modern carbon” (pMC) value that scientists use to estimate carbon ages for carbon-containing materials.
Carbon-14 (14C) doesn’t decay linearly, but instead decays fast at first, then more slowly later, according to a predictable pattern that can be expressed in units called a half-life. Given the relatively short 14C half-life of 5,730 years, organic materials purportedly older than 100,000 years (nearly 18 half-lives) should contain absolutely no detectable 14C. However, coal, diamonds, and even dinosaur bones contain amounts of 14C at levels detectable by modern instruments.[i] Carbon dating of historical objects of known age is sometimes accurate back to about 1,000 BC, as verified by historical records.[ii]
Carbon-14 dating begins with sound, repeatable science when researchers record isotope ratios. So the method itself is not the issue—it’s the assumptions that are made when the raw isotope ratio gets converted to calendar years that carbon dating becomes unreliable and inaccurate, especially on very old artifacts. While carbon dating can in fact return somewhat accurate ages for items that are a couple thousand years old (see discussion and endnotes below), too many evolutionary assumptions accompany carbon dates for items into the deeper past. Several unknown factors can seriously impact carbon ratios. Just a partial list of these factors includes:
Factor 1: Forest fires. Massive forest fires can change 14C/12C ratios much in the same way that volcanic eruptions have.[iii] Do we have a complete record of forest fires dating back thousands of years?
Factor 2: Atomic activity/releases. Atomic bomb testing doubled the amount of 14C in the 1950s and 1960s. Professor Nalini Nadkarni, an ecologist at The Evergreen State College in Washington stated that this testing caused: “a tremendous spike of 14C — actually 100 percent more 14C coming into the atmosphere than what we’d had previous to those atom bomb tests.”[iv] Researchers have found clever ways to normalize measurements to pre-bomb levels, but these extra complications may add more uncertainty to radiocarbon-based age assignments.
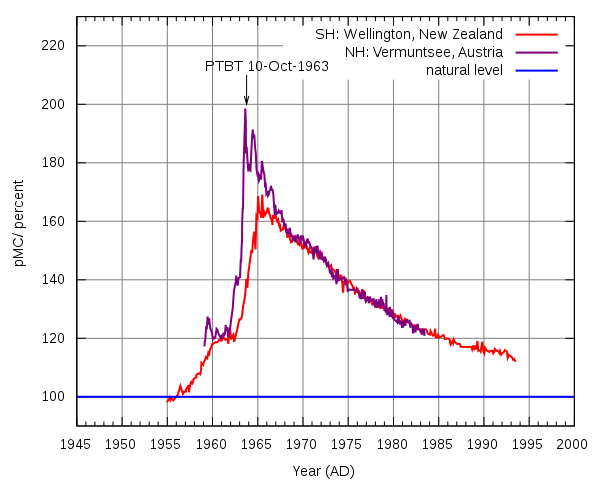
Factor 3: Volcanic eruptions. When volcanoes erupt, they eject enormous amounts of carbon into the air. Because geological carbon does not have much 14C, the 14C/12C ratio in the area gets diluted. In one case, it even made living plants appear to be 1,000 years old![vi] How would a recent past of high volcanism, as shown by ancient lava fields, ash falls, and dead volcanoes, have affected ancient carbon isotope ratios?
Factor 4: Industrialization (past burning of coal). It is widely accepted that the mass burning of coal during the industrial revolution released an enormous amount of 12C into the air, which changed the 14C/12C ratio in the atmosphere. Tree-ring studies can give some insight into the 14C/12C ratio before the industrial revolution, and modern carbon dating takes this into account by running experimental measurements through a calibration formula.[vii] But how do we know what the ratio was like thousands of years ago? We simply don’t. The dating system hangs on these types of assumptions![viii]
Factor 5: Solar flares. Several studies have shown: 1) significant solar flares have occurred in the past, and 2) these flares have an impact on carbon levels in the atmosphere. For example, in AD 774–775 there was an increase of 1.2% in the 14C content of tree rings, which was about 20 times as high as the background rate of variation.[ix] This “spike” was followed by a decline that lasted several years. The cause of this difference is thought to be a solar flare, as the same signal is found in 14C in tree rings around the world, including Germany, Russia, the United States, and New Zealand.[x] Other researchers have noted similar findings.[xi] Do we know whether other solar flares like this occurred thousands of years ago?
Factor 6: The Reservoir Effect. Heavy or light carbon atoms can become trapped, or at least concentrated, in “carbon reservoirs” where carbon isotopes do not quickly mix with the atmosphere.[xii] As a result, some modern deep ocean organics show a carbon age of 1,500 carbon years, for example. Nearby limestone can also affect carbon isotope concentrations, giving false ages—or at least ages that need even more corrections.
Factor 7: Partial pressure. Geologic indicators show that atmospheric CO2 levels were much higher in the past.[xiii]This would have diluted the apparent 14C concentration, again making artifacts look older than they are.
Factor 8: Magnetic field. Several thousand years ago, Earth’s magnetic field may have been twice as strong as today, assuming today’s decay rate.[xiv] This would have slowed the rate at which cosmic radiation generates 14C in the upper atmosphere, disrupting the basis for 14C of very old objects.
Factor 9: Fossil fuels (current and future). A research paper published in the Proceedings of the National Academy of Sciences[xv] led to upheaval in the radioisotope dating field. The study’s findings were spread by numerous science news outlets because they reveal emerging challenges to the reliability of 14C dating based on the impact of burning fossil fuels. For example, Scientific American’s take on the article was: “A T-shirt made in 2050 could look exactly like one worn by William the Conqueror a thousand years earlier to someone using radiocarbon dating if emissions continue under a business-as-usual scenario. By 2100, a dead plant could be almost identical to the Dead Sea scrolls, which are more than 2,000 years old.”[xvi]
Factor 10: Disequilibrium. A critical assumption used in 14C dating has to do with the ratio of 14C to 12 To compute 14C “ages” today, this ratio has to be stable and constant. If this assumption is not true, 14C dating will give incorrect dates. If the amount of 14C being produced in the atmosphere is equal the amount being removed, the atmosphere is in a steady state (also called “equilibrium”). If this is not true, the ratio is not steady, or in a state of disequilibrium.
Dr. Willard Libby, the founder of the 14C dating method, assumed the ratio of 14C and 12C to be constant. His reasoning was based on a belief in deep time and evolution. In his original work, however, he noted that the atmosphere did not appear to be in equilibrium. His calculations showed that if the earth started with no 14C in the atmosphere, it would take up to 30,000 years to build up to a steady state (equilibrium): “If the cosmic radiation has remained at its present intensity for 20,000 or 30,000 years, and if the carbon reservoir has not changed appreciably in this time, then there exists at the present time a complete balance between the rate of disintegration of radiocarbon atoms and the rate of assimilation of new radiocarbon atoms for all material in the life-cycle.”[xvii]
Dr. Libby’s concern has turned out to be very real. Studies since Libby’s original work have shown the 14C / 12C is notconstant: “The Specific Production Rate (SPR) of 14C is known to be 18.8 atoms per gram of total carbon per minute. The Specific Decay Rate (SDR) is known to be only 16.1 disintegrations per gram per minute.”[xviii] If it takes about 30,000 years to reach equilibrium and 14C is still out of equilibrium, the earth must not be very old.
When scientists attempt to stretch the results of carbon dating back many thousands of years, do they inadvertently violate any of the assumptions discussed above? How can we know without being there to measure the various factors to see if the assumptions hold true?
There is actually some solid evidence that many of the assumptions discussed above do not hold true. A key study conducted in 1989 by the British Science and Engineering Research Council (BSERC) arose over concern about the practice of carbon dating. Many results continued to come back with varying dates for various artifacts of known ages (i.e., artifacts which could be reliably dated using written history). So BSERC decided to conduct an international blind test on the practice of carbon dating itself. The test was conducted by sending dated artifacts of “known age” to 38 of the world’s leading radiocarbon testing laboratories. The results of the study confirm that something is amiss:[xix]
The British Science and Engineering Research Council (which funded the installation of the C14 apparatus at Oxford) ran a series of tests in 1989 with 38 laboratories involved worldwide. As a consequence, the council has insisted this year (1990) on new quality-control measures, by which checks are made with standard reference materials of known age. Of the mass spectrometry technique used at Oxford, Dr. Baxter reports: ‘It came out very badly in the survey, even when dating samples as little as 200 years old.’ Only 7 out of 38 laboratories produced satisfactory results, and the margin of error with artefacts of known age was two or three times greater than the technique’s practitioners claim. Nature (the magazine which published details of the original C14 experiment) has now published a demonstration that the radiocarbon technique is not only unsound but also outdated. The Geological Observatory of Columbia University in New York has proved that the C14 results given in past years are in error by as much as 3,500 years in dating fossils, artefacts and events of the past 40,000 years, and the further back we go in time, the greater the error. Dr. Fairbanks of the observatory staff points out that since the C14 dating depends on the ever-variable quantity of C14 in the atmosphere produced by cosmic rays, any alteration of that production either by nature, or by the solar system, or by man-made interference (such as thermo-nuclear bombs) must cause a collapse of the whole hypothesis. He quotes the significant underestimation of the age of ancient objects and states that in a large number of tests C14 failed consistently, the samples being far older than the C14 findings showed. (emphasis added)
How can carbon dating be regarded as scientifically reliable and accurate when 0 of 38 laboratories “achieved a correct date, even with plus or minus tolerances, and many were off by thousands of years”? Do we know about all the forest fires and volcanic eruptions that have occurred in the distant past? Atomic activity? Solar flares and cycles? Earth’s magnetic field? There are so many assumptions required to journey into the distant past—it’s a better idea to trust the Creator, who was there, than the words of secular scientists.
A final factor to consider when it comes to carbon dating is the worldwide Flood described in Genesis 6–9, plus the recent Ice Age that followed right after the Flood. Noah’s Flood would have uprooted and buried entire forest systems, decreasing the release of 12C into the atmosphere through the decay of vegetation. Creation scientists have investigated this, and believe the Flood explains why most dinosaur bones typically cluster between 17,850 to 49,470 radiocarbon years.[xx]
[i] See Paul Giem, Carbon-14 Content of Fossil Carbon, Origins 51 (2001): 6-30; J. Baumgardner, Andrew Snelling, D.R. Humphreys, and Steve Austin, Measurable 14C in Fossilized Organic Materials: Confirming the Young Earth Creation-Flood Model. In Ivey, H (editor) Proceedings of the Fifth International Conference on Creationism, 2003, 127–142. Pittsburgh, PA: Creation Science Fellowship (see also: www.icr.org/article/young-earth-creation-flood-14c/); J. Baumgardner, 14C evidence for a recent global flood and a young earth. In Vardiman, L., Snelling, A.A. and Chaffin, E.F. (editors), Radioisotopes and the Age of the Earth: Results of a Young-Earth Creationist Research Initiative, 2005, pp. 587–630. (El Cajon, CA: Institute for Creation Research and Chino Valley, AZ: Creation Research Society). (See also: www.icr.org/article/carbon-14-evidence-for-recent-global).
[ii] G. Faure, Principles of Isotope Geology, 2nd ed., (New York: John Wiley & Sons, 1986): 391.
[iii] K. J. Heckman, H. Campbell, H. Powers, B. Law, C. Swanston. “The Influence of Fire on the Radiocarbon Signature and Character of Soil Organic Matter in the Siskiyou National Forest, Oregon, USA.” Fire Ecology 9 (2) (2013): 40–56.
[iv] Robert Krulwich, “How A-Bomb Testing Changed Our Trees.” August 25, 2010 (www.npr.org/templates/story/story.php?storyId=96750869) (February 3, 2016).
[v] Image Credit: Wikipedia: https://it.wikipedia.org/wiki/File:Radiocarbon_bomb_spike.svg (January 26, 2017)
[vi] Sheridan Bowman, Radiocarbon Dating. (London: British Museum Press, 1995), p. 24–27.
[vii] Tree ring dating (dendrochronology) has been used in an attempt to extend the calibration of carbon-14 dating earlier than historical records allow, but this depends on temporal placement of fragments of wood from trees that have been dead for ages using carbon-14 dating. For example, the Bristlecone Pines in California were dated to be 4,700+ years old by counting the tree rings, which is within the age brackets of the Flood. However, research conducted on the same type of tree shows that seasonal effects can cause multiple rings (up to five) to grow in the same year. It is likely that the world following the Flood would have been much wetter with fewer contrasting seasons until after the Ice Age, which could explain the apparent date of the tree based on counting its rings. When scientists build calibration models for radiocarbon dating that extend back many thousands of years, they attempt to build tree ring chronologies by “cross-matching” tree ring patterns of pieces of dead wood found near living trees. This process relies on circular reasoning because it assumes that the “carbon clock” can be moved backwards in time in a straight line, and
the Flood greatly disrupted carbon ratios in the earth, as well as the atmosphere that produces the ratios of radioactive and stable carbon. Carbon dating today assumes that the system has been in equilibrium for many thousands of years. However, the Flood buried large quantities of organic matter containing stable carbon (12C) changing the 14C/12C ratios. Further, tree ring patterns are not unique (like fingerprints). Dr. Batten (Batten, Don. “Tree ring dating (dendrochronology).” Creation.com: www.creation.com/tree-ring-dating-dendrochronology(February 10, 2016): “There are many points in a given sequence where a sequence from a new piece of wood matches well (note that even two trees growing next to each other will not have identical growth ring patterns). D.K. Yamaguchi, “Interpretation of cross-correlation between tree-ring series.” Tree Ring Bulletin, 46 (1986): 47–54: Yamaguchi recognized that ring pattern matches are not unique. The best match (using statistical tests) is often rejected in favor of a less exact match because the best match is deemed to be ‘incorrect’ (particularly if it is too far away from the carbon-14 ‘age’). So the carbon ‘date’ is used to constrain just which match is acceptable. Consequently, the calibration is a circular process and the tree ring chronology extension is also a circular process that is dependent on assumptions about the carbon dating system (see: B. Newgrosh, “Living with radiocarbon dates: a response to Mike Baillie.” Journal of the Ancient Chronology Forum 5:59–67, 1992.).
[viii] G. Faure, Principles of Isotope Geology, 2nd ed. (New York: John Wiley & Sons, 1986), p. 391; Ken Ham, Andrew Snelling, and Carl Weiland, The Answers Book, (El Cajon, CA.: Master Books, 1992): 68.
[ix] F. Miyake, K. Nagaya, K. Masuda, T. Nakamura, “A signature of cosmic-ray increase in AD 774–775 from tree rings in Japan,” Nature 486 (7402) (2012): 240–242.
[x] I.G. Usoskin, “The AD 775 cosmic event revisited: The Sun is to blame,” Astronomy & Astrophysics 552 (1) (2013): L3.
[xi] Minze Stuiver, “Variations in Radiocarbon Concentration and Sunspot Activity,” J. Geophysical Research 66 (1961): 273–76.
[xii] R.E. Taylor and O. Bar-Yosef. Radiocarbon Dating: An Archaeological Perspective, 2nd Ed. (Left Coast Press, Walnut Creek, CA, 2014), pp. 31–32; 150–155.
[xiii] Ibid., 45–46.
[xiv] J. Baumgardner, “14C evidence for a recent global flood and a young earth.” In L. Vardiman, Andrew Snelling, and E.F. Chaffin (editors), Radioisotopes and the Age of the Earth: Results of a Young-Earth Creationist Research Initiative.(El Cajon, CA: Institute for Creation Research and Chino Valley, AZ, 2005): 618. Creation Research Society. (www.icr.org/article/carbon-14-evidence-for-recent-global) (January 26, 2017).
[xv] Heather D Graven, “Impact of fossil fuel emissions on radiocarbon,”
Proceedings of the National Academy of Sciences (August 2015), 112 (31): 9542–9545.
[xvi] Von Kaenel, Camille. “Fossil Fuel Burning Obscures Radiocarbon Dates
Increasing atmospheric carbon from burned fossil fuels will make historic dating more difficult,” ClimateWire: www.scientificamerican.com/article/fossil-fuel-burning-obscures-radiocarbon-dates/ (July 21, 2015).
[xvii] W. Libby, Radiocarbon Dating, University of Chicago Press, Chicago, Illinois, 1952, 8.
[xviii] C. Sewell, “Carbon-14 and the Age of the Earth,” 1999. www.rae.org/pdf/bits23.pdf (November 5, 2018).
[xix] Charles Foley, “Dating the Shroud,” The Tablet: International Catholic News Weekly, (July 7, 1990): 13.
[xx] Brian Thomas and Vance Nelson, “Radiocarbon in Dinosaur and Other Fossils,” Creation Research Society Quarterly, 51 (4) (2015): 299–311.







