[Originally published as Creationists Let Facts Speak for Themselves in Mainstream Science Publication]
Joseph Deweese has so far managed to continue as a mainstream scientist while openly professing his belief in creation. He is simultaneously an associate professor of Biochemistry at Lipscomb University and an adjunct professor at Vanderbilt University School of Medicine.
Deweese has published in various top science journals (including Nature) on the Topoisomerase family of enzymes. He (with yours truly) recently published a paper in the journal FASEB (Federation of American Societies for Experimental Biology) in connection with the conference on Experimental Biology in Orlando, Florida, this past April.
(For the record, aside from Deweese, I do not know my co-authors’ views on creation, so I cannot speak to whether they agree with my characterizations in the headline.)
Although Joe’s publications in the mainstream make no mention of the Creator, in other venues he defends his creationist views with evidence from biochemistry.
What Makes Topoisomerases Special?
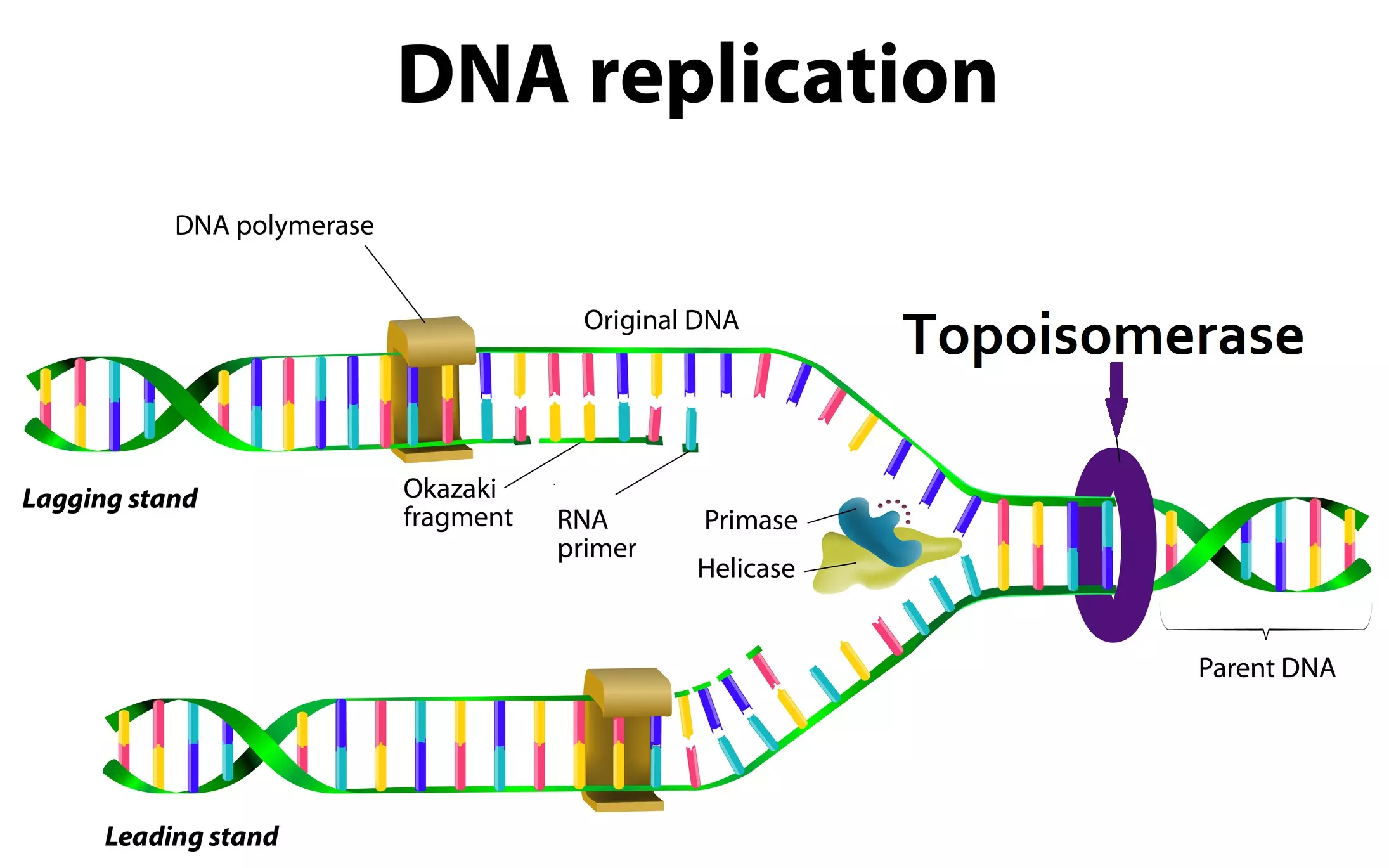
Topoisomerases are a family of enzymes that manipulate the topology of DNA. The Topoisomerase IIA enzymes help to “unwind” DNA. During unwinding, while one part of the DNA strand unwinds, other parts would become tighter and tighter, and tangle unless cut and reconnected periodically during the unwinding operation. Watch this video on YouTube to see how the enzyme works.
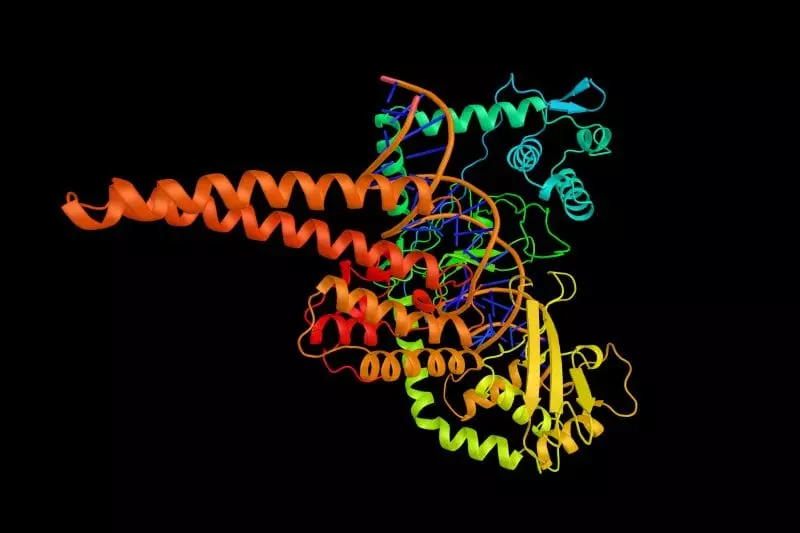
Here is a diagram of the topoisomerase Type IIA enzyme. The top and bottom domains are identical and are coded from the same gene, but they have to connect to each other to make a workable unit.
Note how the two identical copies nicely dock with each other to make a working machine. It is not a trivial task to design the right sequence of amino acids such that two topoisomerase polypeptides make the right fold in the first place. But they also have to create locations where the copies can connect to each other in order to form a working machine (a homodimer), as in the diagram.
The really amazing thing is that not only do the two identical pieces connect to each other to make a working unit, they act as moving parts in a process that accomplishes the following:
- Detect and locate DNA that needs to be untangled (in order to relieve topological strain)
- Cut the DNA using energy from ATP (an ATP site is provided on the topoisomerase)
- Untangle the DNA after cutting
- Re-connect the DNA strands where it was cut

A Mechanism Unreachable by Evolution
Evolutionary biologists believe that such enzymes can evolve naturally, but rarely do they address the mechanical feasibility of evolving such machines by aimless natural processes, such as natural selection.
One can see at a glance some of the issues of forming such a machine from scratch by random mutation alone. But neither can this enzyme form incrementally via natural selection. What good is a proto-topoisomerase that cuts DNA randomly? Or one that cuts DNA and then doesn’t stitch back together the cut strands? Or one that cuts DNA and doesn’t untangle it? Or one that does all the above but can’t sense and then locate that DNA that needs to be untangled?
As suggested above, a further evolutionary problem is that human Type II topoisomerases are formed as two identical parts (a homodimer), but in bacteria such topoisomerses are made of 4 parts from 2 copies from two different genes, making it a “hetero-tetramer.” This makes no sense from an evolutionary standpoint, but it makes total sense in intelligent design.
The notion that biology is designed to resist interpretations of common descent is articulated in Walter ReMine’s book The Biotic Message. His book provided much inspiration for me to persist in searching for evidence of creation when many times I was on the verge of giving up. However, it says in Proverbs 25:2, “It is the glory of God to conceal a matter, it is the glory of kings to search out a matter.” If an Intelligent Designer wanted to assure those willing to see the truth that life could not have been evolved by common descent, but also wanted to conceal this fact to those willfully blind to seeing miracles of creation, then enzymes like topoisomerase are one example. This is especially true since it took space age science to unravel the layers upon layers of design that God has concealed for his glory for several thousand years, but now allows men (kings) in the present day to search the truth out to their glory.
Piling on the Complexity
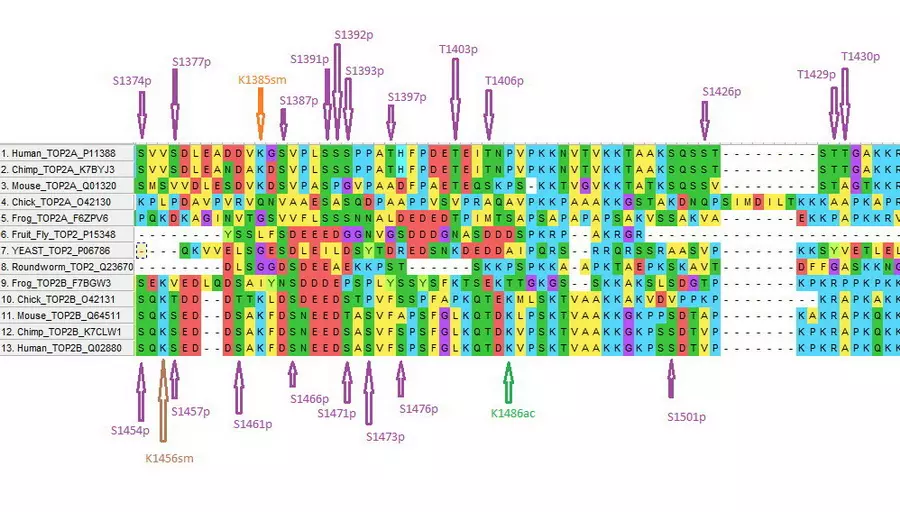
Finally, as reported in our abstract and poster at the Orlando conference, even beyond the problems for evolution mentioned above, in humans, the topoisomerases have an additional layer of immense sophistication: the post-translational modifications (PTMs).
In our Abstract, we describe the possible consequences of PTMs. We show that 191 of the 1,531 amino acids in Topoisomerase IIA are subject to PTMs – known as phosphorylation, ubiqutination, acetylation, methylation, and sumoylation.
In fact, “read”, “write”, and “erase” are the very words biologists themselves have adopted from computer scientists to describe the processing of PTMs.
A suite of machines that perform the “read”, “write” and “erase” modifications has to be recruited to modify the topoisomerases based on cell type and cell phase. In other words, different cells in the body will have different PTMs depending on their type, and PTMs will vary depending on their stage in the cell duplication cycle. This level of complexity boggles the mind. It requires a large suite of specialized molecular machines to manage all the post-translational modifications, in addition to many other machines for reading and translating the genes and folding the polypeptides into the proper shape, then connecting them. Unless all these accessory enzymes are built correctly and perform their jobs correctly, the topoisomerase cannot do its vital task.
But even with all that we’ve discovered, we’ve only scratched the surface of how all of this complexity works!
Topoisomerases are a good example of why we “are fearfully and wonderfully made” (Psalm 139:14).
For more, see Dr. Deweese’s interview discussing this enzyme with David Rives